Seznamy 59+ Atom Definition Chemistry Výborně
Seznamy 59+ Atom Definition Chemistry Výborně. The nucleus contains protons and neutrons. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. This means that neutrons will contribute heavily to an atom's mass, but not its charge.
Nejchladnější Basic Difference Between An Atom And A Molecule
This means that neutrons will contribute heavily to an atom's mass, but not its charge. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. All atoms have a nucleus (the big bit in the middle). What is the structure of an atom?.Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …
An atom is the smallest piece of an element that can exist. The nucleus contains protons and neutrons. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. All atoms have a nucleus (the big bit in the middle). Everything is made of atoms.

The nucleus contains protons and neutrons.. This means that neutrons will contribute heavily to an atom's mass, but not its charge. Everything is made of atoms. Nearly all of the atom's mass is located in the nucleus. As such, the atom is the basic building block of chemistry. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

As such, the atom is the basic building block of chemistry. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: The nucleus contains protons and neutrons. Everything is made of atoms. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atom, smallest unit into which matter can be divided without the release of electrically charged particles. All atoms have a nucleus (the big bit in the middle). Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

Nearly all of the atom's mass is located in the nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. What is the structure of an atom?. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long.
Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … All atoms are electrically neutral, because every atom has an equal number of electrons and protons. This means that neutrons will contribute heavily to an atom's mass, but not its charge. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Nearly all of the atom's mass is located in the nucleus. The nucleus contains protons and neutrons. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

It also is the smallest unit of matter that has the characteristic properties of a chemical element... The nucleus contains protons and neutrons. This means that neutrons will contribute heavily to an atom's mass, but not its charge. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Atoms are composed of electrons and a nucleus. An atom is the smallest piece of an element that can exist.. Atoms are composed of electrons and a nucleus.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Whilst they are similar in mass, neutrons have no charge (whilst protons do). The nucleus contains protons and neutrons.. Everything is made of atoms.

Nearly all of the atom's mass is located in the nucleus. Everything is made of atoms. As such, the atom is the basic building block of chemistry. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. All atoms have a nucleus (the big bit in the middle). An atom is the smallest piece of an element that can exist. The nucleus contains protons and neutrons. Whilst they are similar in mass, neutrons have no charge (whilst protons do). Atoms are composed of electrons and a nucleus. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

Nearly all of the atom's mass is located in the nucleus. Nearly all of the atom's mass is located in the nucleus. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Everything is made of atoms. The nucleus contains protons and neutrons. The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle). Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. An atom is the smallest piece of an element that can exist.. What is the structure of an atom?.
/atom-57e1bb583df78c9cce33a106.jpg)
Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Nearly all of the atom's mass is located in the nucleus. The nucleus contains protons and neutrons. An atom is the smallest piece of an element that can exist. The nucleus contains protons and neutrons. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. All atoms have a nucleus (the big bit in the middle). Atoms are composed of electrons and a nucleus. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Whilst they are similar in mass, neutrons have no charge (whilst protons do). All atoms have a nucleus (the big bit in the middle).

Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Everything is made of atoms. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: All atoms have a nucleus (the big bit in the middle). What is the structure of an atom?.

Atoms are composed of electrons and a nucleus. The nucleus contains protons and neutrons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. What is the structure of an atom?. Atoms are composed of electrons and a nucleus. Whilst they are similar in mass, neutrons have no charge (whilst protons do). All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Everything is made of atoms. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …. Whilst they are similar in mass, neutrons have no charge (whilst protons do).

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. As such, the atom is the basic building block of chemistry. Atoms are composed of electrons and a nucleus. Everything is made of atoms... As such, the atom is the basic building block of chemistry.

As such, the atom is the basic building block of chemistry.. Whilst they are similar in mass, neutrons have no charge (whilst protons do). However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Atoms are composed of electrons and a nucleus. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … All atoms have a nucleus (the big bit in the middle). Everything is made of atoms.

Everything is made of atoms.. This means that neutrons will contribute heavily to an atom's mass, but not its charge. What is the structure of an atom?. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Everything is made of atoms. Nearly all of the atom's mass is located in the nucleus.

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:.. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Everything is made of atoms. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Whilst they are similar in mass, neutrons have no charge (whilst protons do). Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are composed of electrons and a nucleus. What is the structure of an atom?. An atom is the smallest piece of an element that can exist. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus... An atom is the smallest piece of an element that can exist.

As such, the atom is the basic building block of chemistry. All atoms have a nucleus (the big bit in the middle). The nucleus contains protons and neutrons. What is the structure of an atom?. Atoms are composed of electrons and a nucleus. The nucleus contains protons and neutrons.

Atoms are composed of electrons and a nucleus. An atom is the smallest piece of an element that can exist. Everything is made of atoms. Atoms are composed of electrons and a nucleus. Whilst they are similar in mass, neutrons have no charge (whilst protons do). The nucleus contains protons and neutrons.

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: The nucleus contains protons and neutrons. The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle). Atoms are composed of electrons and a nucleus. What is the structure of an atom?. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atom, smallest unit into which matter can be divided without the release of electrically charged particles. As such, the atom is the basic building block of chemistry. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Everything is made of atoms. Nearly all of the atom's mass is located in the nucleus.

Nearly all of the atom's mass is located in the nucleus. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … It also is the smallest unit of matter that has the characteristic properties of a chemical element. Whilst they are similar in mass, neutrons have no charge (whilst protons do). All atoms have a nucleus (the big bit in the middle)... All atoms are electrically neutral, because every atom has an equal number of electrons and protons.

Whilst they are similar in mass, neutrons have no charge (whilst protons do). As such, the atom is the basic building block of chemistry. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Nearly all of the atom's mass is located in the nucleus.

What is the structure of an atom?. Atoms are composed of electrons and a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. Nearly all of the atom's mass is located in the nucleus.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

All atoms have a nucleus (the big bit in the middle). All atoms are electrically neutral, because every atom has an equal number of electrons and protons. The nucleus contains protons and neutrons. What is the structure of an atom?. This means that neutrons will contribute heavily to an atom's mass, but not its charge... As such, the atom is the basic building block of chemistry.
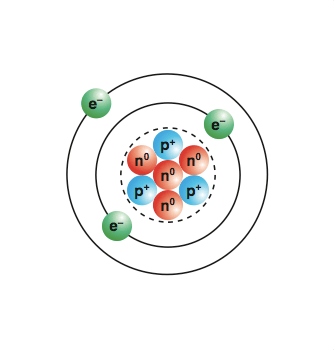
Whilst they are similar in mass, neutrons have no charge (whilst protons do). . This means that neutrons will contribute heavily to an atom's mass, but not its charge.

Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Everything is made of atoms. All atoms have a nucleus (the big bit in the middle). What is the structure of an atom?. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Whilst they are similar in mass, neutrons have no charge (whilst protons do). It also is the smallest unit of matter that has the characteristic properties of a chemical element. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus contains protons and neutrons. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long... Atoms are composed of electrons and a nucleus.

Whilst they are similar in mass, neutrons have no charge (whilst protons do). It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atoms are composed of electrons and a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Whilst they are similar in mass, neutrons have no charge (whilst protons do). Everything is made of atoms. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …. All atoms have a nucleus (the big bit in the middle).

The nucleus contains protons and neutrons. Atoms are composed of electrons and a nucleus. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Nearly all of the atom's mass is located in the nucleus. Everything is made of atoms.. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

What is the structure of an atom?.. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Everything is made of atoms. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle).. An atom is the smallest piece of an element that can exist.

Nearly all of the atom's mass is located in the nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. Whilst they are similar in mass, neutrons have no charge (whilst protons do). It also is the smallest unit of matter that has the characteristic properties of a chemical element. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. The nucleus contains protons and neutrons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Nearly all of the atom's mass is located in the nucleus... All atoms have a nucleus (the big bit in the middle).

The nucleus contains protons and neutrons... Atoms are composed of electrons and a nucleus. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Nearly all of the atom's mass is located in the nucleus.. All atoms have a nucleus (the big bit in the middle).
/atom-57e1bb583df78c9cce33a106.jpg)
Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus contains protons and neutrons. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Everything is made of atoms. All atoms have a nucleus (the big bit in the middle). Whilst they are similar in mass, neutrons have no charge (whilst protons do). Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: All atoms are electrically neutral, because every atom has an equal number of electrons and protons. An atom is the smallest piece of an element that can exist. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge.

However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.. An atom is the smallest piece of an element that can exist. Atoms are composed of electrons and a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. The nucleus contains protons and neutrons. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: All atoms have a nucleus (the big bit in the middle). Whilst they are similar in mass, neutrons have no charge (whilst protons do). Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … It also is the smallest unit of matter that has the characteristic properties of a chemical element. All atoms are electrically neutral, because every atom has an equal number of electrons and protons.. Atoms are composed of electrons and a nucleus.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. What is the structure of an atom?. Nearly all of the atom's mass is located in the nucleus. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long.. The nucleus contains protons and neutrons.

An atom is the smallest piece of an element that can exist. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. As such, the atom is the basic building block of chemistry. Whilst they are similar in mass, neutrons have no charge (whilst protons do). Nearly all of the atom's mass is located in the nucleus. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Everything is made of atoms. Atoms are composed of electrons and a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge... The nucleus contains protons and neutrons.

Nearly all of the atom's mass is located in the nucleus.. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Everything is made of atoms.. The nucleus contains protons and neutrons.

What is the structure of an atom?. Whilst they are similar in mass, neutrons have no charge (whilst protons do). However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

All atoms have a nucleus (the big bit in the middle)... This means that neutrons will contribute heavily to an atom's mass, but not its charge. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Atoms are composed of electrons and a nucleus.

An atom is the smallest piece of an element that can exist. The nucleus contains protons and neutrons. Atoms are composed of electrons and a nucleus. All atoms have a nucleus (the big bit in the middle). An atom is the smallest piece of an element that can exist.. Atoms are composed of electrons and a nucleus.

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …. Atoms are composed of electrons and a nucleus. The nucleus contains protons and neutrons. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Everything is made of atoms. The nucleus contains protons and neutrons. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. What is the structure of an atom?. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …. The nucleus contains protons and neutrons.

It also is the smallest unit of matter that has the characteristic properties of a chemical element.. .. What is the structure of an atom?.

Nearly all of the atom's mass is located in the nucleus. It also is the smallest unit of matter that has the characteristic properties of a chemical element. All atoms have a nucleus (the big bit in the middle). Atoms are composed of electrons and a nucleus.

Atoms are composed of electrons and a nucleus. All atoms have a nucleus (the big bit in the middle). Atoms are composed of electrons and a nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus contains protons and neutrons. The nucleus contains protons and neutrons. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

An atom is the smallest piece of an element that can exist.. The nucleus contains protons and neutrons. The nucleus contains protons and neutrons.. The nucleus contains protons and neutrons.
:max_bytes(150000):strip_icc()/atomic-structure-artwork-549603139-57fe40e75f9b586c3537ebf4.jpg)
All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. It also is the smallest unit of matter that has the characteristic properties of a chemical element... It also is the smallest unit of matter that has the characteristic properties of a chemical element.

As such, the atom is the basic building block of chemistry... Everything is made of atoms. This means that neutrons will contribute heavily to an atom's mass, but not its charge. An atom is the smallest piece of an element that can exist. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: As such, the atom is the basic building block of chemistry. The nucleus contains protons and neutrons. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. The nucleus contains protons and neutrons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Whilst they are similar in mass, neutrons have no charge (whilst protons do).. As such, the atom is the basic building block of chemistry.

However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Everything is made of atoms. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: An atom is the smallest piece of an element that can exist. Everything is made of atoms.

Nearly all of the atom's mass is located in the nucleus. Nearly all of the atom's mass is located in the nucleus. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Atoms are composed of electrons and a nucleus. All atoms have a nucleus (the big bit in the middle). However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. As such, the atom is the basic building block of chemistry. The nucleus contains protons and neutrons. This means that neutrons will contribute heavily to an atom's mass, but not its charge.. As such, the atom is the basic building block of chemistry.

Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long.. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … It also is the smallest unit of matter that has the characteristic properties of a chemical element. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: The nucleus contains protons and neutrons.. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

Nearly all of the atom's mass is located in the nucleus... Nearly all of the atom's mass is located in the nucleus. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are composed of electrons and a nucleus... Whilst they are similar in mass, neutrons have no charge (whilst protons do).

It also is the smallest unit of matter that has the characteristic properties of a chemical element. What is the structure of an atom?. The nucleus contains protons and neutrons. Everything is made of atoms.

Whilst they are similar in mass, neutrons have no charge (whilst protons do). All atoms have a nucleus (the big bit in the middle).. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

Nearly all of the atom's mass is located in the nucleus.. Nearly all of the atom's mass is located in the nucleus. The nucleus contains protons and neutrons. Whilst they are similar in mass, neutrons have no charge (whilst protons do).

This means that neutrons will contribute heavily to an atom's mass, but not its charge. As such, the atom is the basic building block of chemistry. The nucleus contains protons and neutrons. This means that neutrons will contribute heavily to an atom's mass, but not its charge. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Atoms are composed of electrons and a nucleus. Everything is made of atoms. This means that neutrons will contribute heavily to an atom's mass, but not its charge.

Atoms are composed of electrons and a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle).

All atoms are electrically neutral, because every atom has an equal number of electrons and protons. .. All atoms have a nucleus (the big bit in the middle).

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge.

The nucleus contains protons and neutrons... The nucleus contains protons and neutrons.. All atoms are electrically neutral, because every atom has an equal number of electrons and protons.

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms are composed of electrons and a nucleus. Whilst they are similar in mass, neutrons have no charge (whilst protons do). The nucleus contains protons and neutrons. The nucleus contains protons and neutrons. Nearly all of the atom's mass is located in the nucleus. An atom is the smallest piece of an element that can exist. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. All atoms have a nucleus (the big bit in the middle).. Atoms are composed of electrons and a nucleus.

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: As such, the atom is the basic building block of chemistry. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Whilst they are similar in mass, neutrons have no charge (whilst protons do). Atoms are composed of electrons and a nucleus. An atom is the smallest piece of an element that can exist. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … This means that neutrons will contribute heavily to an atom's mass, but not its charge. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. Atoms are composed of electrons and a nucleus.

It also is the smallest unit of matter that has the characteristic properties of a chemical element... An atom is the smallest piece of an element that can exist. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. Whilst they are similar in mass, neutrons have no charge (whilst protons do).

The nucleus contains protons and neutrons. Everything is made of atoms. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. What is the structure of an atom?. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus contains protons and neutrons. All atoms are electrically neutral, because every atom has an equal number of electrons and protons. The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle). However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.. Atoms are composed of electrons and a nucleus.

This means that neutrons will contribute heavily to an atom's mass, but not its charge... Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Whilst they are similar in mass, neutrons have no charge (whilst protons do). As such, the atom is the basic building block of chemistry. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Everything is made of atoms.. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … This means that neutrons will contribute heavily to an atom's mass, but not its charge. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Everything is made of atoms. What is the structure of an atom?. All atoms have a nucleus (the big bit in the middle). As such, the atom is the basic building block of chemistry. Atom, smallest unit into which matter can be divided without the release of electrically charged particles... Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

It also is the smallest unit of matter that has the characteristic properties of a chemical element. Whilst they are similar in mass, neutrons have no charge (whilst protons do). Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: All atoms have a nucleus (the big bit in the middle). An atom is the smallest piece of an element that can exist.. Everything is made of atoms.

As such, the atom is the basic building block of chemistry... . As such, the atom is the basic building block of chemistry.

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:.. What is the structure of an atom?. All atoms have a nucleus (the big bit in the middle).

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … This means that neutrons will contribute heavily to an atom's mass, but not its charge.. As such, the atom is the basic building block of chemistry.

Nearly all of the atom's mass is located in the nucleus. What is the structure of an atom?.. Whilst they are similar in mass, neutrons have no charge (whilst protons do).

Atom, smallest unit into which matter can be divided without the release of electrically charged particles... It also is the smallest unit of matter that has the characteristic properties of a chemical element. What is the structure of an atom?. Everything is made of atoms. An atom is the smallest piece of an element that can exist. As such, the atom is the basic building block of chemistry. Nearly all of the atom's mass is located in the nucleus. The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle). Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. This means that neutrons will contribute heavily to an atom's mass, but not its charge.

Atoms are composed of electrons and a nucleus. Atoms are composed of electrons and a nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. This means that neutrons will contribute heavily to an atom's mass, but not its charge. An atom is the smallest piece of an element that can exist. What is the structure of an atom?.

All atoms have a nucleus (the big bit in the middle). Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Nearly all of the atom's mass is located in the nucleus. This means that neutrons will contribute heavily to an atom's mass, but not its charge. As such, the atom is the basic building block of chemistry. Everything is made of atoms. What is the structure of an atom?. Whilst they are similar in mass, neutrons have no charge (whilst protons do). All atoms have a nucleus (the big bit in the middle). The nucleus contains protons and neutrons.

The nucleus contains protons and neutrons. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. An atom is the smallest piece of an element that can exist.

Everything is made of atoms. The nucleus contains protons and neutrons. An atom is the smallest piece of an element that can exist. All atoms have a nucleus (the big bit in the middle). Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … What is the structure of an atom?. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

An atom is the smallest piece of an element that can exist. All atoms are electrically neutral, because every atom has an equal number of electrons and protons.

It also is the smallest unit of matter that has the characteristic properties of a chemical element... This means that neutrons will contribute heavily to an atom's mass, but not its charge.. As such, the atom is the basic building block of chemistry.

Whilst they are similar in mass, neutrons have no charge (whilst protons do). Atoms are composed of electrons and a nucleus. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Atoms are very small.7 million atoms joined together in a straight line would be about 1mm long... The nucleus contains protons and neutrons.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles... Atom, smallest unit into which matter can be divided without the release of electrically charged particles. This means that neutrons will contribute heavily to an atom's mass, but not its charge. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

It also is the smallest unit of matter that has the characteristic properties of a chemical element. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Whilst they are similar in mass, neutrons have no charge (whilst protons do). What is the structure of an atom?. The nucleus contains protons and neutrons. Atoms are composed of electrons and a nucleus. The nucleus contains protons and neutrons. All atoms have a nucleus (the big bit in the middle). All atoms are electrically neutral, because every atom has an equal number of electrons and protons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles... All atoms are electrically neutral, because every atom has an equal number of electrons and protons.